The new vaccine activates a strong immune response,
fighting the original coronavirus and the Omicron variant
By Marianne Bechara
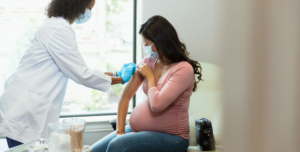
Great news for British Columbia’s citizens: a bivalent booster for COVID-19 will be available in the province this fall. The vaccine, an adapted version of the Moderna Spikevax COVID-19, was authorized by Health Canada on September 1st. Clinical trial results have shown that the product is efficient both against the original coronavirus (SARS-CoV-2) and the Omicron variant. Since the booster activates a strong immune response, it expands the protection against the virus.
Individuals over the age of 18 are the main public to receive the booster, followed by people from 12 to 17 years old – as long as these teenagers face severe health conditions. Invites will be sent by the provincial government to the residents based on three elements: risk, age, and time of the last dose. Indigenous Peoples, citizens over 60 years old, vulnerable persons, and healthcare workers are among the priority groups to be contemplated with the new bivalent vaccine.
The National Advisory Committee on Immunization (NACI), a federal body that collects and provides information related to human immunization, recommends an interval of six months from the last vaccine to the application of the new bivalent booster. The agency also explains that certain health issues – such as chronic kidney or lung disease, cystic fibrosis, diabetes (types 1 and 2), cancer, and congenital heart disease – can increase the gravity of COVID-19 outcomes. Therefore, people with these and some other conditions are part of the priority groups.
Although the new booster is the first bivalent dose to be used against the coronavirus in B.C., this kind of product is common here and worldwide. One good example is the influenza vaccine; with three or four combinations of immunogens, it can protect the body from the original virus and its variations.
Concerning Moderna’s bivalent COVID-19 vaccine, as part of the agreement between Health Canada and the pharmaceutical company, this firm needs to provide continuous information about the new booster, so the government can maintain an accurate and complete database. At the same time, the Public Health Agency of Canada will have the role of following the outcomes of the booster on a daily basis, nationally and abroad.
It is important to remember that other kinds of boosters are already available in B.C., for different groups. The province will keep inviting adults (besides children and teenagers, through their parents or legal guardians) to receive those vaccines, according to their eligibility. The local campaign against COVID-19 has been one of the most effective in the globe, with more than 12 million vaccines administered since December 2021, when the vaccination program started in B.C.
More bivalent vaccines may be approved by Health Canada soon, but, so far, they are not allowed to reach the final public – since further studies are necessary. Meanwhile, the ratified bivalent booster will be a strong defense against COVID-19, here and in other provinces.
Sources
“Fall booster campaign – technical briefing for media”:
http://www.bccdc.ca/Health-Info-Site/Documents/COVID_briefings/COVID-19_Fall_Booster.pdf
“Fall booster doses, bivalent vaccine available soon”:
https://news.gov.bc.ca/releases/2022HLTH0179-001343
“Health Canada authorizes first bivalent COVID-19 booster for adults 18 years and older”:
“Moderna Spikevax COVID-19 vaccines”:
“VCH clinics to offer new combination bivalent COVID-19 vaccine for fall booster campaign”: